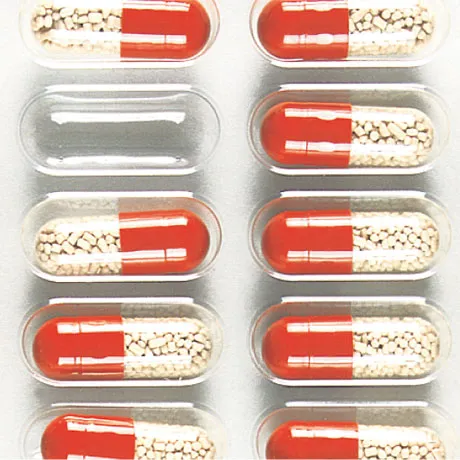
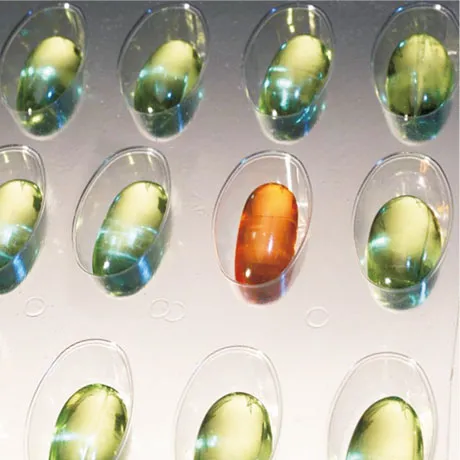
Quality Control System for Tablets
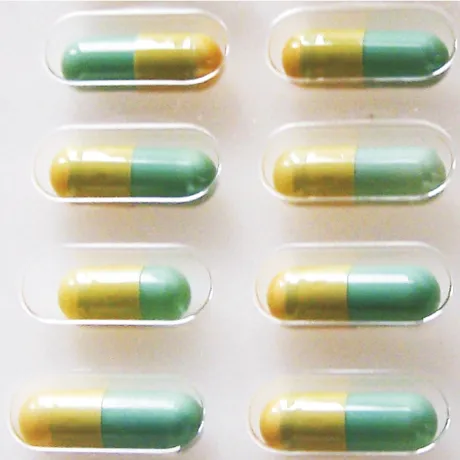
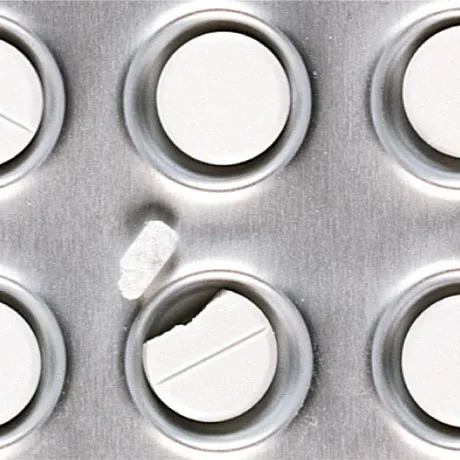
Various defects in tablets and the contents of blisters, including color, shape, and dimensions of the tablets, as well as their positioning in the blister, are examinable. Additionally, the adequacy or excess of the contents and the correct placement in the packaging can be identified. In this way, manufacturers ensure that tablets are always filled according to specifications, and only flawless products exit the production line.
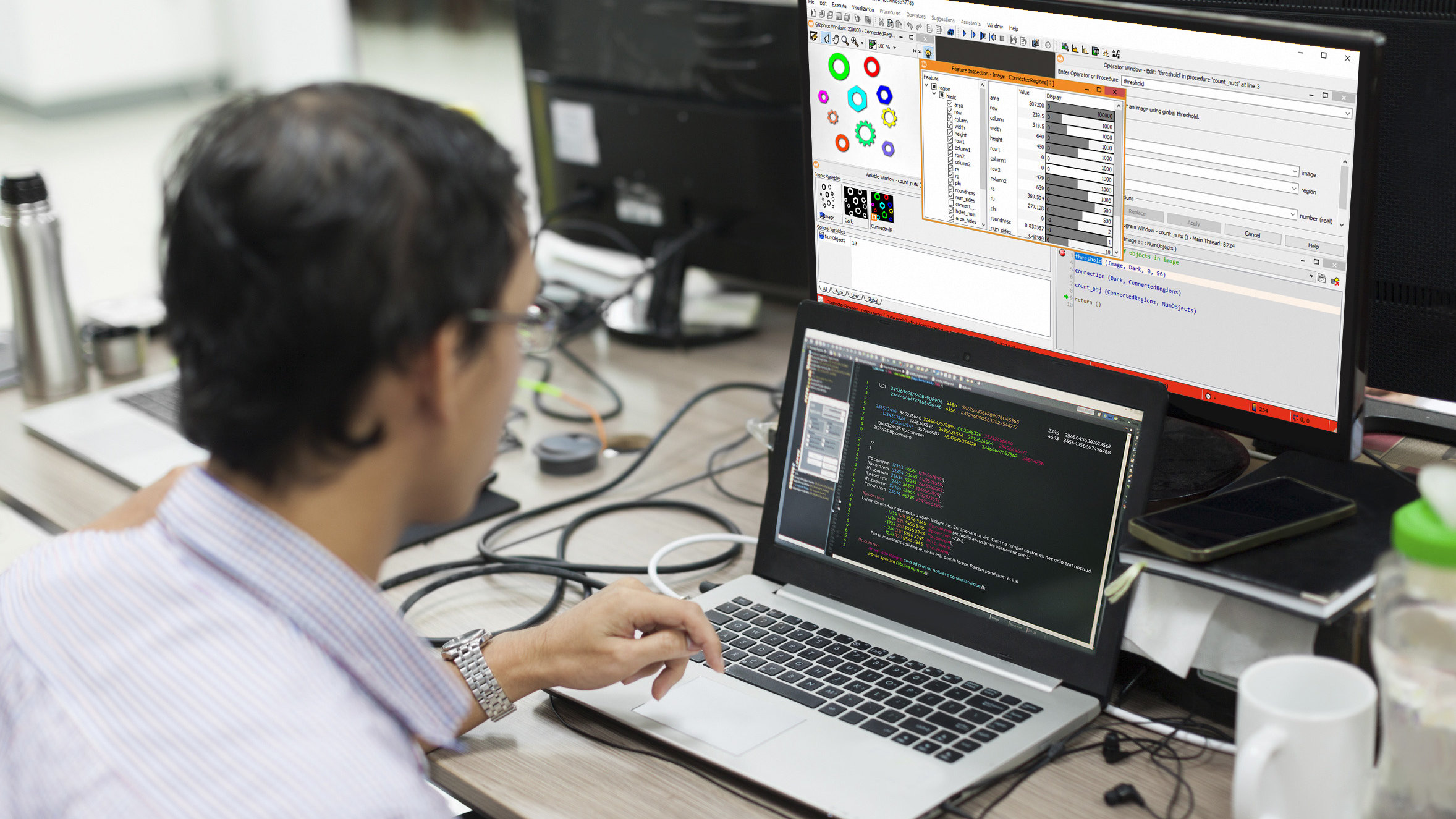
Companies operating across various industries worldwide constantly face numerous challenges. Intense competition in most markets means that the most crucial aspect is surpassing customer expectations in the shortest possible time. One of the best ways to achieve this is by enhancing quality control and leveraging the latest visual inspection technologies to ensure that raw materials and final products are precisely as they should be.